Building materials are substances or components that are used in construction and other building projects to create structures, infrastructure, and other built environments.
These materials can vary widely in their properties, characteristics, and uses, and they play a crucial role in determining the strength, durability, safety, and aesthetics of the final structure.
Building materials can be natural or synthetic, and they are often chosen based on factors such as cost, availability, environmental impact, and intended use.
Table of Contents
- Some common types of building materials include:
- The primary function of Building Materials
- Building Materials and Engineering
- Selecting Building Materials for Engineered Work
- Types of Building Materials
- Types of Polymers
- Ceramics
- Types of ceramics
- Composites Materials
- Semiconductors
- Popular On Website for Engineers Right Now!
Some common types of building materials include:
- Concrete: A versatile material made from a mixture of cement, water, aggregates (such as sand and gravel), and additives. It’s used for a wide range of applications, including foundations, walls, floors, and more.
- Brick: Manufactured from clay and hardened by heat, bricks are commonly used for walls and other structural elements due to their durability and strength.
- Wood: A traditional building material known for its versatility and natural aesthetic. It’s used in various forms for framing, flooring, roofing, and finishing.
- Steel is a solid and durable metal often used in structural components, such as beams and columns. It’s famous for its high strength-to-weight ratio.
- Glass: Used for windows, doors, facades, and interior partitions. It allows light to enter and provides visibility while maintaining separation between spaces.
- Plastic and Composite Materials: These materials offer lightweight, flexible, and corrosion-resistant options for various applications in construction.
- Stone: Natural stone materials like granite, marble, and limestone are used for decorative and structural purposes in buildings.
- Cement Board: Used as a base for tiling in wet areas like bathrooms and kitchens, cement board is water-resistant and provides a stable surface.
- Insulation Materials: These materials, such as fiberglass, foam, and cellulose, are used to improve energy efficiency by reducing heat transfer through walls, roofs, and floors.
- Roofing Materials: Shingles, tiles, metal panels, and other materials are used to protect buildings from weather elements.
- Adhesives and Sealants: These are used to bond materials together, seal gaps, and prevent water infiltration.
- Masonry Materials: Besides bricks, other masonry materials include concrete blocks, stone veneer, and clay tiles.
- Bituminous Materials: Asphalt and tar-based materials are used for roads, pavements, and roofing.
- Gypsum and Drywall: Gypsum is used for interior wall finishes, and drywall is a widely used interior wall paneling material.
- Composite Materials: Combinations of different materials designed to offer specific characteristics like strength, lightweight, and corrosion resistance.
These are just a few examples of the many building materials available. The choice of materials depends on factors like the type of structure, intended use, budget, environmental considerations, and local building codes.
The primary function of Building Materials
The primary function of building materials is to withstand applied loading without breaking or exhibiting excessive deflection. The major classifications of building materials include metals, polymers, ceramics, and composites.

Building materials serve various functions in construction and building projects, each contributing to the overall performance, durability, safety, and aesthetic appeal of the structures. Here are some key functions of building materials:
- Structural Support: Many building materials, such as concrete, steel, and wood, provide the essential strength and support needed to create stable and safe structures. These materials form the framework and load-bearing elements of buildings.
- Insulation: Insulation materials, such as foam, fiberglass, and cellulose, help regulate the temperature inside buildings by minimizing heat transfer. They keep buildings warmer in cold weather and cooler in hot weather, thus enhancing energy efficiency.
- Protection: Building materials like roofing materials, exterior cladding, and sealants protect the structure from environmental elements such as rain, wind, UV radiation, and temperature fluctuations.
- Soundproofing and Acoustics: Some materials are chosen for their ability to dampen sound and improve acoustic performance within buildings, creating a comfortable and quiet environment.
- Fire Resistance: Fire-resistant materials, like fire-rated drywall and fireproof coatings, slow the spread of fire within a building, giving occupants more time to evacuate and reducing property damage.
- Aesthetics and Design: Materials like glass, stone, wood, and decorative finishes contribute to a structure’s visual appeal and design aesthetics. They help create a desired architectural style and ambiance.
- Waterproofing: Materials such as waterproof membranes, sealants, and coatings are applied to areas exposed to moisture, like basements and bathrooms, to prevent water infiltration and damage.
- Durability: Building materials are chosen for their ability to withstand wear and tear over time. Durable materials ensure that the structure maintains its integrity and appearance for an extended period.
- Environmental Sustainability: Sustainable building materials, like recycled materials and eco-friendly products, reduce the environmental impact of construction projects by conserving resources and reducing waste.
- Load Distribution: Materials like steel beams and columns distribute the load of the structure, ensuring that the weight is appropriately supported and transferred to the foundation.
- Flexibility and Adaptability: Some materials, such as modular systems and lightweight panels, allow for easy modifications and alterations in building designs to accommodate changing needs.
- Ease of Installation: Building materials that are easy to work with, install, and manipulate save time and labor costs during construction.
- Electrical and Thermal Conductivity: Materials like copper and aluminum are chosen for their excellent electrical conductivity, while others, like insulated panels, are used to manage thermal conductivity.
- Anti-Corrosion: Materials like galvanized steel and certain coatings are used in corrosive environments to prevent degradation due to exposure to moisture and chemicals.
- Health and Safety: Building materials should meet safety standards to ensure that they don’t pose health risks to occupants due to factors like toxicity or allergens.
Each building material’s function contributes to the overall performance and longevity of structures while addressing specific needs and challenges posed by the environment, design, and intended use of the building.
Also Read: Corrosion in Reinforced Concrete: Cause, Prevention and More
Building Materials and Engineering
The types of building materials available to engineers are vast and may possess widely different properties, e.g., asphalt (bitumen), steel, concrete, rubber, timber, stone, plastic, ceramic, etc.
Engineers use all of these materials, but their character, chemical, and physical properties differ, ranging from soft, solid, semi-solid, and easily deforming metals such as lead to tool steel, which is complex and challenging.
However, the different engineering fields focus on their subset of products and systems, etc. The Engineering Materials are highly usable by the following Engineers;
- Civil engineers
- Mechanical engineers
- Electrical engineers etc
For construction purposes, engineers or designers design products and systems, make them, and monitor their usage to ensure the proper use of all materials.
The engineer needs to select suitable and available materials to meet the requirements of the design and of the construction at hand.
Building Materials, however, should possess high specific quality with correct properties, first for production and subsequently for service, such that they will not fail in use. They must be well-selected to produce durability and stability.
As there are numerous materials available, it is impossible, if not complex, for the engineer to possess details of all of them, but it is essential to understand the fundamental principles that control their properties.
Materials are common in one property, and they are all composed of atoms. These atoms are composed of three particles which are:
Also Read: Landfill: General Overview You Should Know In 2023
Protons
Critical characteristics of protons include:
- Charge: Protons carry a positive charge of +1 elementary charge. This positive charge is balanced by an equal number of negatively charged electrons in a neutral atom.
- Mass: The mass of a proton is approximately one atomic mass unit (AMU). An amu is a unit used to express the relative masses of atomic particles. The mass of a proton is roughly 1836 times greater than the mass of an electron.
- Nuclear Stability: Protons and neutrons contribute to the stability of the atomic nucleus. Protons’ positive charge repels other protons due to their electrostatic repulsion. The presence of neutrons helps counteract this repulsion and contributes to holding the nucleus together.
- Strong Nuclear Force: The strong nuclear force is the fundamental interaction that binds protons and neutrons together within the atomic nucleus. This force is responsible for overcoming the electromagnetic repulsion between protons.
- Identity of Elements: The number of protons in an atom’s nucleus is unique to each element and determines its chemical identity. The periodic table is organized based on the number of protons (atomic number) in each element.
- Radioactivity: Certain elements have unstable nuclei, which can lead to the emission of particles or energy. This process, known as radioactivity, can involve the conversion of a proton into a neutron or vice versa.

Neutrons
Some key characteristics of neutrons:
- Charge: Neutrons are electrically neutral, which means they do not carry a positive or negative charge. Unlike protons, they do not contribute to the overall electrical charge of an atom.
- Mass: The mass of a neutron is approximately one atomic mass unit (amu), similar to the mass of a proton. Neutrons are slightly more massive than protons, but their mass is still much greater than that of electrons.
- Nuclear Stability: Neutrons contribute to the stability of the atomic nucleus by counteracting the electrostatic repulsion between positively charged protons. The strong nuclear force, one of nature’s fundamental forces, helps hold neutrons and protons together in the nucleus.
- Isotopes: Different isotopes of an element have the same number of protons (and therefore the same atomic number), but they can have different numbers of neutrons. Varying the number of neutrons in an atom’s nucleus gives rise to different isotopes of an element. Isotopes of an element can have slightly different physical properties, and some isotopes may be radioactive.
- Radioactivity: Neutrons can also influence the stability of atomic nuclei and can trigger nuclear reactions. In some cases, neutrons can be absorbed by nuclei, causing them to become unstable and leading to processes like nuclear fission or nuclear fusion.
- Neutron Sources: Neutrons are used in various applications, including nuclear research, medical treatments, and industrial processes. Neutron sources can be natural, such as certain types of radioactive decay or artificial, created through nuclear reactions.
- Neutron Scattering: Neutron scattering is a technique used to study the atomic and molecular structure of materials. Neutrons interact with atomic nuclei and provide valuable insights into the arrangement of atoms within a substance.
Also Read: The Best Garden Hoses of 2022 (Reviewed)
Electrons
Critical characteristics of electrons:
- Charge: Electrons carry a negative charge of -1 elementary charge. This charge is equal in magnitude but opposite in sign to the positive charge of protons. The number of electrons in an atom is typically equal to the number of protons, ensuring that the atom is electrically neutral.
- Mass: The mass of an electron is significantly smaller than that of protons and neutrons. It is approximately 1/1836th of the mass of a proton or neutron. Due to their small mass, electrons contribute relatively little to the total mass of an atom.
- Energy Levels and Orbitals: Electrons occupy specific energy levels, or electron shells, around the nucleus. Each energy level can hold a certain number of electrons. Within these energy levels, electrons are further arranged into orbitals, which describe the probability distribution of finding an electron within a particular region of space around the nucleus.
- Quantized Energy States: Electrons can only occupy discrete energy levels in an atom, and they can transition between these levels by absorbing or emitting energy in the form of photons. This behavior is fundamental to the emission and absorption of light by atoms.
- Chemical Bonding: Electrons are involved in chemical bonding, where they interact with electrons from other atoms to form bonds. The sharing, transfer, or interaction of electrons is what enables the formation of molecules and compounds.
- Electricity and Conductivity: The movement of electrons constitutes electric current. In conductive materials like metals, electrons are relatively free to move, allowing for the transmission of electrical charge.
- Quantum Mechanics: Electrons exhibit both particle-like and wave-like behavior. Quantum mechanics is the branch of physics that describes the behavior of electrons and other particles on the atomic and subatomic scales.
- Spin: Electrons have an intrinsic spin, a quantum mechanical property, without a classical analog. Spin is related to electrons’ magnetic behavior.
- Electron Cloud: The region around the nucleus where electrons are likely to be found is often called the electron cloud. The cloud represents the probability distribution of electron positions and considers electrons’ wave-like nature.
The behavior in which these particles are assembled in atoms and how atoms are bonded to one another control essentially the final properties of the bulk engineering materials in daily use.
Selecting Building Materials for Engineered Work
Selecting suitable materials for engineered projects is a critical process that involves considering various factors to ensure the success, safety, and longevity of the final product.
Whether you’re designing a building, a machine, a bridge, or any other engineered structure, the choice of materials plays a significant role in determining its performance.
Here’s a step-by-step guide to help you select materials for engineered work:
- Define Project Requirements: Start by understanding the project’s purpose, function, and intended use. What are the load-bearing requirements, environmental conditions, lifespan expectations, and aesthetic considerations?
- Identify Material Properties: List the material properties that are essential for your project. These properties could include strength, durability, corrosion resistance, thermal conductivity, electrical properties, and more.
- Consider Loading Conditions: Analyze the types of loads the structure will experience, such as static loads, dynamic loads, impact loads, and temperature fluctuations. Choose materials that can withstand these conditions.
- Review Codes and Standards: Check for relevant industry standards, codes, and regulations that govern material selection for your specific type of project. Compliance with these standards is crucial for safety and legal reasons. Some standard codes are Indian Standards, British Standards, Chinese Standards, etc.
- Evaluate Environmental Factors: Consider the project’s location and exposure to environmental elements like moisture, chemicals, UV radiation, and temperature variations. Choose materials that can withstand these conditions without deteriorating.
- Assess Cost and Budget: Balance material performance with project budget constraints. Some materials may offer superior properties but come at a higher cost—factor in both material costs and potential long-term savings or costs associated with maintenance.
- Review Availability and Lead Time: Ensure that the chosen materials are readily available and can be sourced within your project’s timeline. Delays in material procurement can impact project schedules.
- Consider Sustainability: Evaluate the environmental impact of your material choices. Opt for materials with lower carbon footprints, recyclability, and reduced resource consumption if possible.
- Explore Material Compatibility: Consider how the selected materials will interact with each other and with any existing structures or components. Compatibility is crucial to prevent corrosion, galvanic reactions, and other issues.
- Test and Prototyping: Depending on the project’s complexity, material testing and prototyping are conducted to verify the performance of chosen materials under realistic conditions.
- Seek Expert Advice: Consult with materials engineers, structural engineers, architects, and other experts in the field to get insights into material suitability and alternatives.
- Document Your Decision: Create a materials selection document outlining the rationale for your choices, including material properties, test results, costs, and compliance with standards.
- Monitor Performance: After the project is complete, continue to monitor the performance of the selected materials over time. This can help inform future material choices and design improvements.
Engineers make their choices partly based on their experience and partly on the technical evidence available in the materials they have at their disposal.
The engineer’s experience here allows for scientific analysis of all the aspects of an engineering problem.
Many of the results of the scientific approach are available to the engineer in the form of property values and the behavior of materials.
Engineers should understand the factors that affect the properties of materials and be able to:-
- Identify economic requirements such as overall costs.
- Select more materials that are perfect for the intended work.
- Identify service requirements such as Minimum strength, Wear resistance, Weathering resistance, and Corrosion resistance.
- Carry out different tests of different types of ascertaining their quality as may be required to suit the purpose.
- Avoid service conditions that might damage the materials.
- Be capable of modifying the properties of materials as the need may arise to come up with better materials with competitive properties to suit the requirement.
The engineering requirements of materials mean what is expected from the materials so that they can be successfully used for making engineering works, e.g. Strength, wear resistance, etc.
The task of a civil engineer is to judge and make good decisions to achieve the highest level of competence. Decisions and selections must be based on the best available information.
Also Read: Structural Engineer | All You Need To Know
Types of Building Materials
There are many materials available to engineers; however, building materials may be grouped into metals, ceramics, polymers, composites, and semiconductors.
Metals are a class of materials characterized by their excellent conductivity, strength, malleability, and durability.
They play a crucial role in various industries, including construction, manufacturing, transportation, electronics, and more.
Metals are composed of closely packed atoms arranged in a crystalline structure, and they typically have a shiny appearance due to their ability to reflect light.
Metals are inorganic substances composed of one or more metallic elements and may also contain some non-metallic elements.
Examples of metals are Fe, Cu, Zn, etc, and examples of non-metals are O, C, S, etc. Metallic materials have crystalline structures in which atoms are arranged in a well-defined order.
Metals have high electrical and thermal conductivity and can be polished to a high luster. The attribute of good conductivity is that electronics have greater freedom of movement in metals than in polymers and ceramics.
Also Read: Structural Design Basis | General Guidelines
Types of Polymers
Polymers are a class of materials composed of large molecules made up of repeating subunits called monomers. They are known for their versatility, lightweight nature, and wide range of applications across various industries.
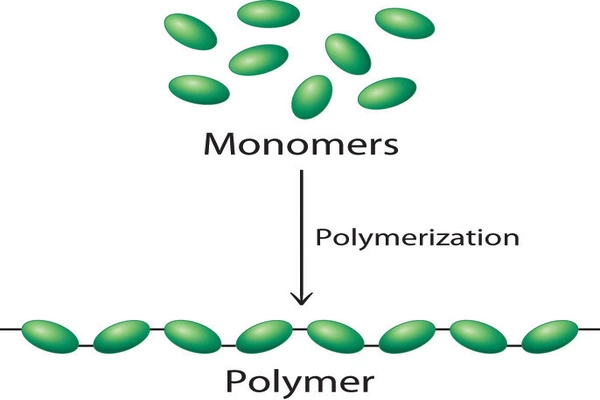
Polymers can be found in everyday products like plastics, rubber, fibers, and more complex materials. They are synthesized through polymerization, a chemical process that links monomers together to form long chains or networks.
Here are some fundamental properties and characteristics of polymers:
- Diversity: Polymers encompass a vast array of materials with different properties and uses. They can be rigid or flexible, transparent or opaque, and conductive or insulating, depending on the specific type and arrangement of monomers.
- Lightweight: Many polymers have relatively low densities, making them lightweight compared to metals and ceramics. This property is advantageous in applications where weight is a consideration.
- Flexibility: Polymers can be engineered to be flexible and elastic, allowing them to withstand deformation without breaking. Rubber, for example, is a flexible polymer commonly used for tires and other products.
- Low Thermal Conductivity: Polymers generally have lower thermal conductivity compared to metals, which makes them suitable for applications where heat insulation is essential.
- Electrical Insulation: Many polymers are good insulators of electricity, making them useful in applications where electrical conductivity must be minimized.
- Corrosion Resistance: Unlike metals, polymers are generally resistant to corrosion and do not require additional coatings to protect against environmental exposure.
- Ease of Processing: Polymers can be molded, extruded, and shaped into various forms using relatively simple processes. This allows for efficient and cost-effective manufacturing.
- Color and Transparency: Polymers can be produced in a wide range of colors and degrees of transparency, making them suitable for applications where aesthetics are essential.
- Chemical Resistance: Some polymers exhibit excellent resistance to chemicals, acids, and bases. This property is essential in applications involving chemical storage or transport.
- Biocompatibility: Certain polymers are biocompatible, meaning they are well-tolerated by living organisms. These polymers find applications in medical devices, implants, and drug delivery systems.
Common examples of polymers include:
- Polyethylene (PE): Used in a variety of plastic products, including bottles, bags, and packaging.
- Polypropylene (PP): Known for its strength and chemical resistance, used in containers, automotive parts, and textiles.
- Polyvinyl Chloride (PVC): Used in pipes, cables, clothing, and other applications due to its durability and versatility.
- Polystyrene (PS): Used in packaging, disposable utensils, and foam products like styrofoam.
- Polyester: Used in clothing, textiles, and packaging.
- Nylon: Known for its strength, used in fabrics, ropes, and various industrial applications.
- Polyurethane (PU): Used in foam insulation, furniture, footwear, and coatings.
- Silicone: Known for its high-temperature resistance and biocompatibility, used in medical devices, cookware, and sealants
Polymers have revolutionized modern manufacturing and technology, offering solutions to a wide range of challenges across industries.
Their diverse properties make them suitable for applications in packaging, automotive components, medical devices, electronics, and more.
Also Read: The Communication Skills: All Engineers Need To Know
Ceramics
Ceramics are a wide-ranging group of inorganic materials consisting of compounds of non-metallic and metallic elements; the chief ingredients are clays, sands, and feldspar.
Clay contains Si, Al, and other elements in chemical combinations known as silicates. K, Mg, and Ca compounds are also present, depending on the type of rock from which the clay was formed.
Sand contains silica (SiO) and sometimes feldspar or aluminum potassium silicate.
The main properties of ceramics are:-
- Hard
- Wear resistance
- Strong in compression
- Electrical insulators
- Many of them can withstand very high and are therefore suitable for use as refractory materials in furnaces and kilns.
The main disadvantages of ceramics are:
- Brittleness
- Weak in tension
- Not very tough
These hardness and brittleness are the general attributes of ceramics, which make them more resistant than metal or polymer materials to high temperatures and help serve the environment.
The electronic behavior of constituent atoms is the basis for these characteristics. Metallic elements (atoms) release their outermost electrons, which are received by non-metallic atoms, which retain them; all these develop a strong attraction force to each other.
Types of ceramics
- Traditional Ceramics:
- Clay Ceramics: Includes pottery, porcelain, and earthenware. These ceramics are made from naturally occurring clay minerals and are often used for artistic and functional objects.
- Refractory Ceramics: Designed to withstand high temperatures and harsh conditions, used in furnaces, kilns, and industrial equipment.
- Advanced Ceramics:
- Oxide Ceramics: Composed of metal and oxygen atoms, including materials like alumina (aluminum oxide) and zirconia (zirconium oxide). They have high mechanical strength and excellent electrical insulating properties.
- Non-Oxide Ceramics: Composed of elements other than oxygen, such as carbides, nitrides, and borides. Silicon carbide and silicon nitride are examples of non-oxide ceramics known for their high strength and thermal stability.
- Piezoelectric Ceramics: Exhibit piezoelectric properties, meaning they generate an electric charge when subjected to mechanical stress and are used in sensors, actuators, and transducers.
- Dielectric Ceramics: Have high electrical resistance and are used as insulators in electronic components like capacitors.
- Ferrites: Ceramics with magnetic properties, used in microwave devices, transformers, and magnetic storage media.
- Bioceramics:
- Bioinert Ceramics: Used in medical implants and devices due to their compatibility with biological tissues. Examples include alumina and zirconia implants.
- Bioactive Ceramics: Form chemical bonds with bone tissue, promoting integration. Hydroxyapatite is an example used in bone grafts and coatings for implants.
- Glass-Ceramics:
- Intermediate materials between glass and ceramics, produced by controlled crystallization of glass. They combine the properties of both materials and are used in cookware, dental ceramics, and more.
- Whisker-Reinforced Ceramics:
- These ceramics are strengthened by incorporating whisker-like crystalline materials, such as silicon carbide whiskers, to enhance mechanical properties.
- Ceramic Matrix Composites (CMCs):
- Combinations of ceramics and other materials, such as carbon or silicon carbide fibers, embedded in a ceramic matrix. CMCs offer high strength and temperature resistance and are used in aerospace and high-performance applications.
- Electroceramics:
- Include materials with specific electrical properties, such as piezoelectric ceramics for sensors and actuators, and ferroelectric ceramics used in capacitors and memory devices.
- Thermal Barrier Coatings:
- Used in high-temperature environments, such as turbine blades in jet engines. These ceramics provide thermal insulation and protect underlying materials from extreme heat.
These are just a few examples of the many types of ceramics available. The choice of ceramic type depends on factors like intended application, required properties, and operating conditions.
Ceramics offers a wide range of options for industries seeking materials with high-temperature stability, mechanical strength, electrical insulating properties, and more.
Also Read: Corrosion in Reinforced Concrete: Cause, Prevention and More
Composites Materials
Composites are made up of two or more materials that may be bonded together as laminae or combined so that one material acts as a matrix surrounding fibers or particles of another.
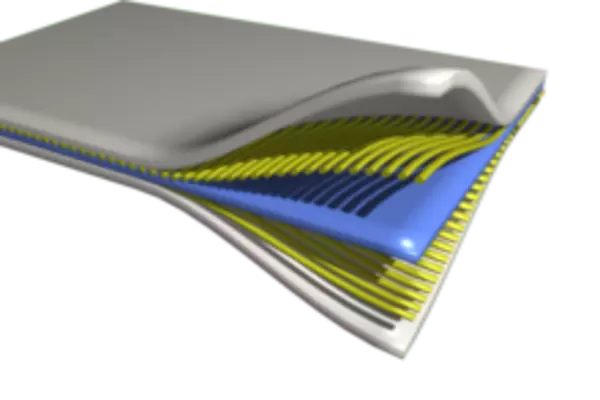
Plywood and concrete reinforced by steel are examples of composites. Types of composite materials are:
- Particulate -this includes concrete where coarse and fine aggregate is bonded in the matrix of cement, high-impact polystyrene reinforced by rubber particles, etc. Ceramics cement is an excellent thermal insulator and thus is used for heat shields in aerospace ships.
- Fibrous The most common fibrous composites are fiber-reinforced thermosetting plastics, which use epoxy and polyester resin as matrix material, here the role of fiber is to carry the greater part of the load and provide stiffness. Carbon fiber-reinforced plastics are very strong, stiff, and light in weight, they are used in aircraft and fishing roads. Kevlar is also used to reinforce rubber in tires.
- Laminated Laminates are layers of material that are bonded together to give improved strength and rigidity. They are divided into:
- Wood laminates- include plywood and laminated chipboard.
- Polymer laminates are made from thermosetting plastic resin and filler material such as paper and cloth. They are used for trays and worktops.
- Polymer-metal combinations-they consist of a low-density lightweight core contained between two high-strength skins. They are commonly used in aircraft due to their lightness and rigidity.
Semiconductors
Semiconductors are a class of materials with electrical properties that lie between those of conductors (materials with high electrical conductivity) and insulators (materials with low electrical conductivity).
They play a fundamental role in modern electronics and technology by serving as the foundation for various electronic components and devices.
Semiconductors are used in everything from microchips and transistors to solar cells and light-emitting diodes (LEDs).
Properties and characteristics of semiconductors:
- Conductivity: Semiconductors have electrical conductivity that is sensitive to external factors like temperature, light, and voltage. At low temperatures, they behave as insulators, but as temperature increases, their conductivity also increases.
- Band Gap: Semiconductors have a band gap, which is the energy gap between the valence band (energy levels occupied by electrons) and the conduction band (energy levels that electrons can move into). The band gap determines whether a semiconductor is a direct or indirect bandgap semiconductor and influences its optical and electrical properties.
- Doping: The electrical properties of semiconductors can be modified through a process called doping. Doping involves introducing impurities into the crystal lattice of the semiconductor to create extra charge carriers (either electrons or holes) and thereby enhance conductivity.
- Electron Mobility: Semiconductors can exhibit different electron mobility values, which describe the speed at which charge carriers move through the material in response to an electric field.
- P-N Junction: By combining a region of a semiconductor with excess electrons (n-type) and a region with a deficit of electrons (p-type), a p-n junction is created. This junction forms the basis for diodes and transistors.
- Transistors: Semiconductors are the building blocks of transistors, which are essential components in electronic circuits. Transistors can amplify signals and act as switches, forming the basis of digital logic.
- Photoconductivity: Some semiconductors become more conductive when exposed to light, a property known as photoconductivity. This property is utilized in devices like solar cells and photodetectors.
- Thermoelectric Effect: Certain semiconductors exhibit the thermoelectric effect, where temperature differences generate a voltage potential. This effect is harnessed for thermoelectric generators and cooling devices.
- Quantum Effects: At the nanoscale, semiconductors can exhibit quantum effects that lead to unique properties and behaviors, such as quantum dots and quantum wells.
Common examples of semiconductors include:
- Silicon (Si): The most widely used semiconductor material in electronics. Silicon-based devices form the backbone of integrated circuits (microchips).
- Germanium (Ge): Another common semiconductor material used in early electronics, but it’s less commonly used today due to its lower operating temperature range compared to silicon.
- Gallium Arsenide (GaAs): Used in high-frequency and high-speed electronic devices, as well as in optoelectronics due to its direct band gap.
- Indium Phosphide (InP): Used in high-speed transistors, photodetectors, and optical communication devices.
- Cadmium Selenide (CdSe) and other Quantum Dots: Used in nanotechnology and as fluorescent markers in biological research.
We hope this article helped you learn about Building Engineering. You may also want to learn about Reinforced Cement Concrete, Theory of Structures, Door Types for Your Modern Home, and Cladding: Types and Considerations.
If you liked this article, please Join WebsiteForEngineers on Telegram, and you can also find us on Pinterest, Twitter, and Facebook.










I need lessons and books
Please sir, bookmark this page and keep coming back for more informative tutorials.